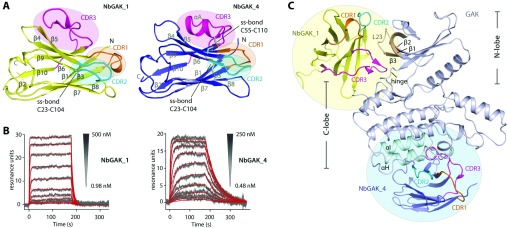
Structure of cyclin G-associated kinase (GAK) trapped in different conformations using nanobodies.
Biochemical Journal
Volume 459, Issue 1, 1 April 2014, Pages 59-69
Chaikuad, A., Keates, T., Vincke, C., Kaufholz, M., Zenn, M.d, Zimmermann, B., Gutiérrez, C., Zhang, R.-G., Hatzos-Skintges, C., Joachimiak, A.f, Muyldermans, S., Herberg, F.W., Knapp, S., Müller, S.
Abstract
GAK (cyclin G-associated kinase) is a key regulator of clathrin-coated vesicle trafficking and plays a central role during development. Additionally, due to the unusually high plasticity of its catalytic domain, it is a frequent ‘off-target’ of clinical kinase inhibitors associated with respiratory side effects of these drugs. In the present paper, we determined the crystal structure of the GAK catalytic domain alone and in complex with specific single-chain antibodies (nanobodies). GAK is constitutively active and weakly associates in solution. The GAK apo structure revealed a dimeric inactive state of the catalytic domain mediated by an unusual activation segment interaction. Co-crystallization with the nanobody NbGAK-4 trapped GAK in a dimeric arrangement similar to the one observed in the apo structure, whereas NbGAK-1 captured the activation segment of monomeric GAK in a well-ordered conformation, representing features of the active kinase. The presented structural and biochemical data provide insight into the domain plasticity of GAK and demonstrate the utility of nanobodies to gain insight into conformational changes of dynamic molecules. In addition, we present structural data on the binding mode of ATP mimetic inhibitors and enzyme kinetic data, which will support rational inhibitor design of inhibitors to reduce the off-target effect on GAK.