Characterization of nanobodies binding human fibrinogen selected by E. coli display
b Research Institute of Biomedical and Health Sciences, Veterinary Faculty, Universidad de Las Palmas de Gran Canaria (UPGC), Arucas, Las Palmas, Canary Islands, Spain
c Centro de Biología Molecular “Severo Ochoa” (Consejo Superior de Investigaciones Científicas—Universidad Autónoma de Madrid), Campus UAM Cantoblanco, Madrid, Spain
Abstract
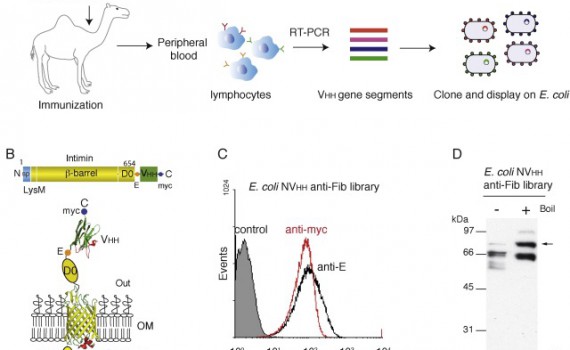
Abnormal levels of fibrinogen (Fib) in blood plasma are associated with several pathological conditions and hence methods for its detection in blood and body fluids are essential. Nanobodies (Nbs) or (VHHs) are single domain antibodies derived from camelids with excellent biophysical and antigen-binding properties, showing great promise in diagnostics and therapy. In this work, we select and characterize high affinity Nbs binding human Fib employing an E. coli cell surface display system based on the fusion of an immune library of VHH domains with the β-domain of Intimin. Bacteria displaying high-affinity Nbs against Fib were selected using magnetic cell sorting (MACS). Specific binding of the selected clones to Fib was confirmed by flow cytometry of E. coli bacteria, as well as by enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) with the purified Nbs. E. coli display also provided an excellent estimation of the affinity of the selected Nbs by flow cytometry analysis under equilibrium conditions, with equilibrium constant (KD) values very similar to those obtained by SPR analysis. Finally, pairwise epitope-scouting studies revealed that the selected Nbs bound distinct epitopes on Fib. The selected Nbs are promising diagnostic tools for determination of human Fib levels.